Titanium nitride is a chemical compound of the two elements titanium and nitrogen. TiN according to its formula is a metallic hard material of typical golden yellow color? The ceramic material is characterized by very high hardness and corrosion resistance, resulting in a number of technical applications. Here are the options for titanium nitride now.
Extraction and presentation

Titanium nitride is usually produced in the form of wafer-thin coatings, more rarely as a ceramic body or as a powder. Production of the elements is possible at temperatures above 1200 ° C, which must be paid to the exclusion of atmospheric oxygen and moisture, which is technically complex.
Another possibility to produce titanium nitride is gas-phase ammonolysis at temperatures above 900 ° C. Here, the titanium tetrachloride contained titanium from the oxidation stage is reduced +4 to +3 in the titanium nitride. As an electron supplier, the nitrogen is made from ammonia. Similar to direct nitriding of titanium, care must be taken to exclude oxygen and moisture.
The following procedures relate to the production of TiN for coating purposes
The direct nitriding of titanium is performed in a KCN / K 2 CO 3 – molten salt. Common processes are case-hardening in the cyanide-containing salt bath (TIDURAN process) and high-pressure nitriding (TIDUNIT process). A protective layer obtained by nitriding generally consists of an approx. 10-micron thick bonding layer and a 50-200 micron thick diffusion layer.
The production of ceramic bodies is difficult since pure TiN has only a low sintering activity due to its high covalent bonding character. Therefore, the compaction of the TiN moldings and the use of sintering additives and external pressure are required. Without this pressure, the ceramics do not achieve the theoretical density and other advantageous properties. However, methods are known which avoid these high pressing pressures by extremely fine, so-called nano-scale powder as a starting material.
Physical Properties
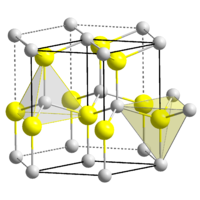
TiN has an intercalation structure and crystallizes in the common salt lattice, whereby the titanium atoms form a face-centered cubic lattice and the small nitrogen atoms are embedded in the octahedral gaps of the base structure. The crystal structure characterizing this metallic hard material exists only in the composite and not in the form of individual molecules, which is reflected in its insolubility in almost all, even the most aggressive solvents.
The extremely high hardness exceeds that of chrome steel, with titanium carbide having a higher degree of hardness. The hardness is 2450 HV (For comparison alumina 2100 HV titanium carbide 3200 HV). TiN has a very high melting point, but no boiling point, as premature decomposition occurs. The material has good friction properties and is therefore of interest for systems with special requirements for lowest wear. The adhesion to other materials is extremely low. In contrast to non-metallic hard materials such as diamond, B 4 C 3 or silicon carbide TiN shows pronounced metallic behavior, such as the conductivity for electric current.
Conclusion
The temperature coefficient of electrical resistance is positive and the magnetic behavior is due to a weak, temperature-dependent para-magnetism characterized. At a temperature of T = 4.86 K TiN is superconducting. TiN has a high reflectivity for infrared radiation. Its reflection spectrum is similar to that of gold. By adding a few atomic percents of amorphous silicon to titanium nitride, extreme changes in mechanical properties (increase in hardness and fracture toughness) can be achieved. The material’s many outstanding technical properties are offset by its brittleness, which is why it is mainly used in the form of very fine coatings.